Corrosion is essentially an electrochemical process, which affects the corrosion of aluminum foil due to internal factors related to the aluminum metal itself, such as the nature, composition, structure and surface state of aluminum, as well as the existence of mechanical deformation and internal stress.
The second is the external factors related to corrosion, such as the composition, concentration, liquid temperature, flow state of the corrosive liquid, and the magnitude and frequency of the impressed current.
Aluminum foil etching cross-section

Low pressure aluminum foil
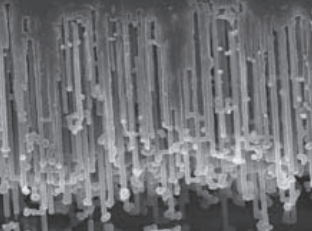
High-pressure aluminum foil
The influence of aluminum foil purity
The purity of aluminum foil is very high, its purity is 99.85%~99.99%, and it contains only a very small amount of magazine. When the standard electrode potential containing metal impurities is positive compared to that of aluminum (it is less likely to lose electrons than aluminum), then it will constitute the cathode of the corroding battery, and the aluminum with a negative potential will be the anode of the corroding battery.
The aluminium is corroded. Therefore, in order to increase the corrosion rate and corrosion rate, trace impurities are sometimes consciously incorporated into the manufacture of aluminum foil.
Influence of mechanical deformation and internal stress
During the machining of aluminum foil in cold rolling and rolling, the aluminum metal structure undergoes irreversible plastic deformation. If the aluminum foil after rolling processing is not annealed, there must be considerable internal stress.
Both stress and deformation can accelerate the corrosion process of metals, and when the stress is evenly distributed, the effect on the corrosion rate is not very large. However, if the stress distribution is uneven, that is, there is a stress concentration, the corrosion process is not only accelerated.
But in many cases, due to the distortion and deformation of the metal lattice, the corrosion in these areas may become crevice corrosion, increasing the corrosion depth, and causing the aluminum foil to corrode and crack. The main method of preventing stress corrosion is annealing to relieve its stress.
The influence of heat treatment
Changing the structure of a metal (or alloy) with a heating method is called heat treatment of the metal. Heat treatment methods include quenching, tempering, and annealing, while aluminum foil is commonly annealed. The so-called annealing is the insulation of the metal at a high temperature for a certain period of time, and then it is slowly cooled.
Heat treatment can not only eliminate the internal stress caused by cold rolling processing of aluminum foil, but also promote certain physical transformations in the crystal structure of metal or alloy, including diffusion (homogenization of the alloy), recrystallization, grain growth, etc. The annealing temperature has a direct effect on the cubic crystal structure content, grain size and impurity precipitation in the aggregate structure.
Generally speaking, the high annealing temperature, the coarse grains of recrystallization, and the increase in the number of cubic grains in the aggregate structure are conducive to the increase of specific volume.
The influence of the pH value of the electrolyte solution
Among the factors that affect the rate of galvanic corrosion, the pH value of the electrolyte solution for corrosion also has a great influence.
The pH value of the solution has two effects on metal corrosion: on the one hand, with the increase of the concentration of hydrogen ions in the solution, that is, the decrease of the pH value, the electrode potential of the cathode or hydrogen electrode (the cathode from which the hydrogen escapes can be regarded as the hydrogen electrode) becomes more positive, which makes the hydrogen ions easier to discharge, promotes the hydrogen depolarization process on the cathode.
Thereby accelerating the corrosion of aluminum, and on the other hand, because the change of pH value also affects the growth or dissolution of the oxide film layer on the metal surface, so it also affects the speed of metal corrosion.
Aluminum is an amphoteric metal that is unstable in both acid and broken solutions, because both aluminum and aluminum oxides will dissolve in both acid and broken solutions.
The composition and concentration of corrosive fluid
In a neutral salt solution such as NaCI in aqueous solution, the rate of corrosion depends on a number of factors, including the solubility of the corrosion product. If the electrode process forms insoluble corrosion products, the corrosion rate is significantly reduced.
For example, the electrolytic corrosion of aluminum foil in neutral table salt, the corrosion product of the anodic process is mainly soluble AlCl3, but a small amount of insoluble AI(OH)3 precipitation may also be generated, and a small amount of hydrochloric acid is usually added to neutralize the insoluble AI(OH)3 in order to maintain a certain corrosion rate.
The influence of corrosion fluid temperature and current density on corrosion rate
In electrolyte solutions, the rate of corrosion generally increases with the temperature of the liquid, because when the temperature increases, the resistivity of the electrolyte solution decreases, and both the anode and cathode processes are accelerated. In addition, the higher the density of the current passing through the foil during corrosion, the faster the rate of corrosion, and vice versa.
