The aluminum foil must also be pre-treated before it can be anodized to form a dielectric film. One of the purposes of pre-treatment is to make the surface state of the aluminum foil as pure as possible, so as not to affect the quality of the formed foil. For example, aluminum foil will form a very thin layer of aqueous oxide film on the surface of the air and need to be removed, so the corrosive foil needs to be cleaned and dried before forming.
The second purpose is to prefabricate a certain thickness of a special oxide film on the surface of the aluminum foil, such as forming a porous oxide film in advance, and then forming a dielectric film between the porous film and aluminum.
The porous oxide film plays a protective role, so that the dielectric film generated by the subsequent formation process is less affected by mechanical damage and CI–, and the effect of stabilizing the film layer is achieved, so that the capacitor has good electrical and temperature resistance characteristics.
There are generally three methods of pretreatment, which can be selected according to the requirements of different foil characteristics, which are briefly described below.
Heat treatment
Aluminum metal is subjected to a high-temperature heat treatment in the atmosphere, and it is obvious that the oxygen in the air will combine with the aluminum to promote the formation of an oxide film on the surface. If the heat treatment temperature exceeds 450 ℃, the growth of the membrane is accelerated, and the grown membrane contains a crystalline oxide film.
This oxide film is formed by heat treatment and then anodized in the formation solution.
The formation mechanism is that the anion OH– or O2- passes through the porous membrane and the thin barrier layer in the middle, and reaches the metal surface to make the barrier oxide film formed.
On the other hand, Al3+ will continue to grow a barrier layer on the side of the liquid surface through the barrier layer, and the pores that originally formed the porous part are buried by oxides (also known as buried hole treatment), so that the outer side of the porous oxide film also generates a barrier layer, which is mostly used for low-pressure oxide films.
The advantage of heat-treated oxide film is that it has high formation efficiency and saves electricity. However, there are also disadvantages:
- At very low voltage, the capacitor has large losses;
- When there is no load at high temperature, the leakage current increases;
- The oxide film is prone to cracking. Because the amorphous film becomes a crystalline film, the density increases, which means that the volume shrinks, causing cracking.
Hydration treatment
For medium and high pressure aluminum foil, it is hydrated with high-temperature boiling water before it is formed. As the name suggests, hydration can save energy by generating a hydrated oxide film, and the hydrated foil will significantly reduce the production of gel-flocculent AI2(OH)3 when formed.
In addition, when the hydrated foil is formed, part of the hydrated oxide film will be converted into a dielectric oxide film, so the dielectric strength of the medium will be improved.
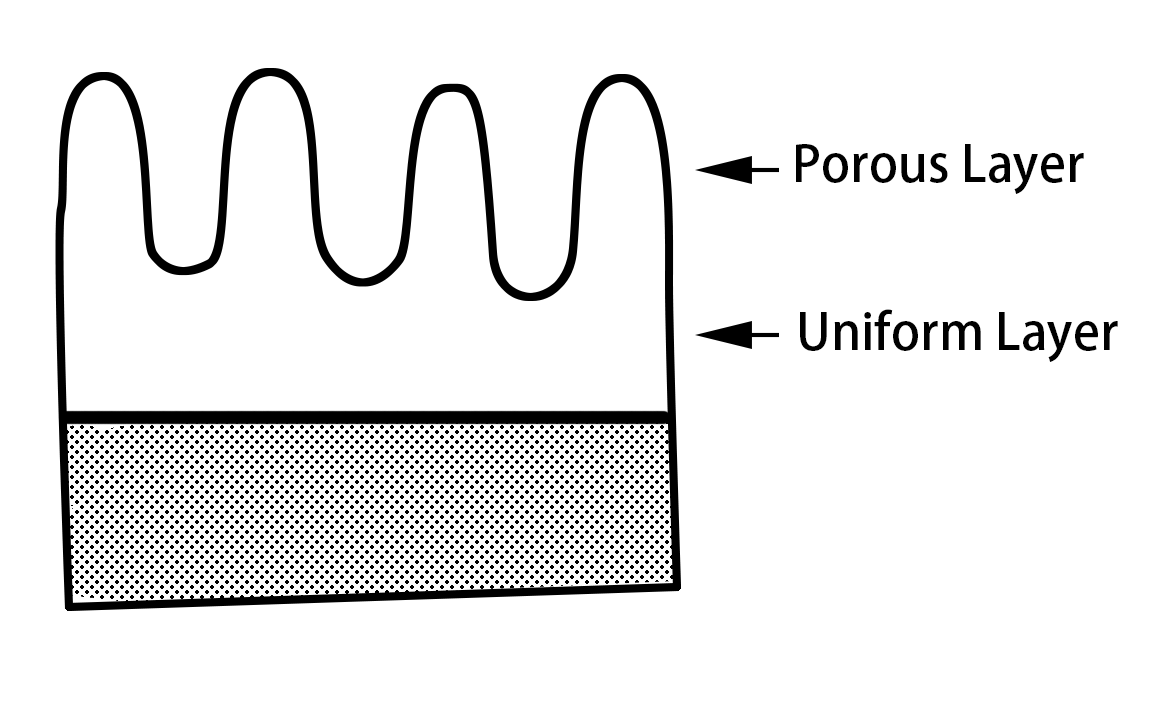
Model diagram of oxide film structure for hydration treatment
The reaction between aluminium and high-temperature water goes through three processes:
- Due to the action of the microcell, the cathode will precipitate H2, so that the anode forms amorphous alumina;
- Amorphous alumina hydrolysis, according to different PH values, can form [AL(OH)]2+,[AL(OH)2]+,[AL(OH)4];
- These ions reach a certain concentration on the surface of aluminium and are formed and precipitated in water as hydrated alumina
The process of its reaction is as follows:
2AL+(3+n)H2O=AL2O3.nH 2O+3H2
Membrane cracking also occurs in membranes treated with hydration treatment, which is caused by shrinkage when it becomes amorphous crystal, which is prone to cracking, and cracking occurs when hydroxide is dehydrated into crystalline oxides.
Form a protective film treatment
The protective film is composed of amorphous AI2O3, which has a thin barrier layer between the aluminum surface and the porous layer, in addition to many small pores. When this aluminum foil is immersed in the formation solution for anodizing, the 02– and OH– ions reach the surface of the aluminum through the barrier layer present inside the pores to grow a new barrier layer, and the Al3+ ions grow a barrier layer at the liquid interface through this barrier layer.
At this point, because the liquid level is located at the bottom of the hole, the hole will be buried from the bottom towards the opening side, so that the porous oxide will bind to the barrier layer.
Generally, the protective film is formed after the formation of the protective film is carried out, and a small part of it is left in the process without completely burying the hole, so that the outer porous layer plays the role of a protective barrier layer. The thickness of the protective layer is thicker for high-pressure foils and thinner for low-pressure foils.
The outermost barrier layer with a large number of small porous protective films can improve the chemical stability of the barrier layer in terms of structure, which can not only prevent deterioration but also improve heat resistance. However, the protective film treatment will dissolve the aluminum foil to some extent, and fill some small holes, resulting in reduced corrosion efficiency.
It should be noted that protective film formation was widely used in the past, and now ordinary electrolytic capacitors are no longer used because it affects the expansion of the surface area of aluminum foil. However, this pre-treatment process is still used to form a protective film for aluminum electrolytic capacitors with harsh environmental conditions or for welding with high-voltage repeated charging and discharging.
